 |
The
global eClinical solutions market size
is expected to reach USD 12.05 million by 2025, according to a new report by
Grand View Research, Inc., progressing at a CAGR of 13.6% during the forecast
period. Increasing research and development activities by biopharma and pharma
companies, rising application of software solutions in clinical trials, and
expanding customer base are anticipated to fuel the demand for eClinical
solutions.
The
eClinical solutions market is estimated to witness phenomenal growth over the
forecast period. Technological advancements in the field of clinical trials
such as electronic data capture and prevalence of Wi-Fi connectivity are
projected to drive the market in coming years. As the demand for tracking and
analyzing clinical data increases, the need for effective clinical solutions
rises. Unmet needs to manage efficient clinical development process are poised
to boost the growth of the market over the forecast period.
Moreover,
digital transformation in the field of clinical trials and shifting preference
towards data centric approach are providing a tremendous push to the market.
Demand for integrated clinical IT solutions is increasing due to massive volume
of data generated during clinical development processes. eClinical solutions
offer single source of information that helps in optimizing the cost by
eliminating redundant data entry and reducing on-site verification and source
data verification rate. Rising awareness regarding these benefits is propelling
the market.
Adoption
of eClinical workflows in trials offer enormous potential in clinical
development processes. These solutions can facilitate decision making in each
stage of development. It also helps in reducing cost & time between
development phase by utilizing seamless designs & identifying failing
compounds. In addition, it offers rapid access to data & patient safety
information, which is helpful in making quick decisions.
Full
research report on eClinical solutions market analysis: https://www.grandviewresearch.com/industry-analysis/eclinical-solutions-market
Further
Key Findings From the Study Suggest:
- CTMS dominated the
product segment in 2016 owing to associated benefits such as centralized
end-to-end management of clinical trial activities, elimination of
reliance on manual processes, real-time status tracking, and maintenance
of multiple databases, which cumulatively improve the overall efficiency
of clinical trials
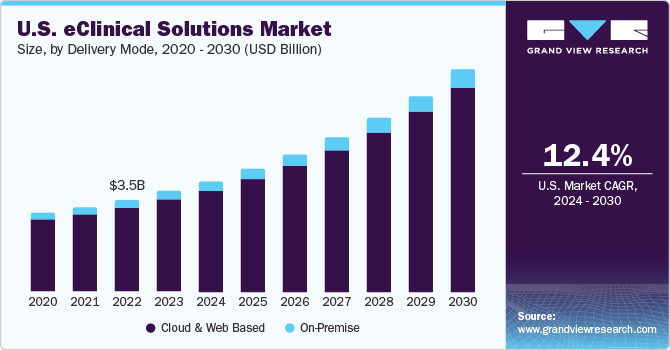
- On-premise
eClinical solutions are most commonly preferred solutions. The preference
for these services is mainly due to complete access to information and
have full control within the premise
- Key market players
are engaged in various strategies such as mergers & acquisitions and
new product development to gain market penetration
- New product
development and strategic alliances including partnership agreements,
promotional activities, and acquisitions are instrumental in keeping
market rivalry high
- For instance, in February
2016, Oracle Health Sciences entered into an agreement with Bayer
Healthcare to provide its clinical e-monitoring solutions, Oracle Siebel
Clinical Trial Management System, to the latter
View
more reports of this category by Grand View Research at: https://www.grandviewresearch.com/industry/healthcare-it
Grand
View Research has segmented the global eClinical solutions market report on the
basis of end-user application and region:
eClinical
Solutions Product Outlook (Revenue, USD Million, 2014 - 2025)
- Electronic Clinical
Outcome Assessment (eCOA)
- Electronic Data
Capture (EDC) and Clinical Data Management Systems (CDMS)
- Clinical Analytics
Platforms
- Clinical Data
Integration Platforms
- Safety Solutions
- Clinical Trial
Management Systems (CTMS)
- Randomization and
Trial Supply Management (RTSM)
- Electronic Trial
Master File (eTMF)
eClinical
Solutions Delivery Mode Outlook (Revenue, USD Million, 2014 - 2025)
- Web-hosted
(On-demand)
- Licensed Enterprise
(On-premise)
- Cloud-based
eClinical
Solutions Clinical Trial Outlook (Revenue, USD Million, 2014 - 2025)
- Phase I
- Phase II
- Phase III
- Phase IV
eClinical
Solutions End-use Outlook (Revenue, USD Million, 2014 - 2025)
- Hospitals
- CROs
- Academic Institutes
- Pharmaceutical
Biopharmaceutical Companies
- Medical Device
Manufactures
eClinical
Solutions, Regional Outlook (Revenue, USD Million, 2014 - 2025)
- North America
- S.
- Canada
- Mexico
- Europe
- K.
- Germany
- France
- Italy
- Spain
- Netherlands
- Sweden
- Denmark
- Asia Pacific
- Japan
- China
- India
- Korea
- Australia
- New Zealand
- Taiwan
- Hong Kong
- Singapore
- Thailand
- Vietnam
- Latin America
- Brazil
- Argentina
- Chile
- Middle East &
Africa
- South Africa
- Saudi Arabia
- A.E
- Egypt
- Qatar
Access
Full Press Release of this Report:
https://www.grandviewresearch.com/press-release/global-eclinical-solutions-market
https://www.grandviewresearch.com/press-release/global-eclinical-solutions-market
About
Grand View Research
Grand
View Research, Inc. is a U.S. based market research and consulting company,
registered in the State of California and headquartered in San Francisco. The
company provides syndicated research reports, customized research reports, and
consulting services. To help clients make informed business decisions, we offer
market intelligence studies ensuring relevant and fact-based research across a
range of industries, from technology to chemicals, materials and healthcare.